Roll up their little sleeves: Israeli infants from six months through kids up to six years will soon be eligible for COVID-19 vaccines.
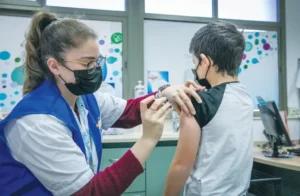
Israeli babies from age six months through age five will soon be able to vaccinate against COVID-19 following a decision by Health Ministry director-general Nachman Ash to accept an enthusiastic recommendation of the team for treatment of pandemics.
The ministry will check on the availability of vaccines in the health funds in the coming weeks; vaccine doses for this age group will be smaller than those for children aged six and up that were approved months ago. The Pfizer and the Moderna vaccines, both approved by the US Food and Drug Administration for babies and small children, are recommended especially for children at risk of serious chronic diseases and those who are undergoing treatment that weakens the immune system. The US Centers for Disease Control and Prevention (CDC) also recommended giving the vaccines to this age group.
The mRNA vaccines made by the two companies have proven to have a high safety profile and to be effective in dealing with the new Coronavirus. Moderna is planning to test its vaccine in children ages three to six months. In younger children, they presumably also get some antibodies against COVID-19 from their mother, but they can disappear over time.
The recommendation was made by the Health Ministry’s team of experts after the vaccine was approved by the FDA and after two professional and in-depth discussions in which a comprehensive overview of the vaccines’ effectiveness and safety was provided to them.
According to the companies’ recommendations, for the Pfizer vaccine, children under age five receive three 3-microgram doses. Children five to 11 receive two 10-microgram doses, while those 12 and up receive two 30-microgram doses, which is the same for adults.
COVID-19 vaccination statistics
For the Moderna vaccine, children under six receive two 25-microgram doses, compared to two 50-microgram doses for ages six to 12, and two 100-microgram doses in ages 12 and up.
Vaccination uptake in young American children has not been high so far. Only about 29% of children ages five to 11 are fully vaccinated, according to the CDC. This compares to a vaccination rate of 60% among kids ages 12 to 17. There are about 18 million children under the age of five in the US.
Pfizer enrolled 4,500 American children in its clinical trials. For those aged five and under, 1,678 children received two doses three weeks apart and then a third dose at least two months after the second dose. The vaccine was well tolerated and there were no new safety issues signaled; the vast majority of side effects were mild or moderate and temporary. they reported. After a third dose, children five and under elicited a strong immune response, and efficacy was 80.3% in preventing symptomatic infection. These preliminary findings were based on 10 symptomatic cases identified seven days after the third dose. The immune response among children under five (measured one month after the third dose) compared favorably to that of two doses among 16- to 25-year-olds. Parts of the studies took place before the Omicron variant was predominant.
Moderna enrolled 11,700 US children aged 6 months to 12 years in their studies, including 6,700 under six years. All children received two doses of the vaccine 28 days apart. According to data from the company, the majority of side effects for the group under six years of age) were mild or moderate and mostly reported after the second dose. No new safety concerns were identified. Interim results showed that the vaccine was 51% effective against symptomatic infection among children ages six months to two years, and 37% effective among those two to five years. In both age groups, two doses compared favorably to the immune response adults ages 18 to 25 had after two doses. The studies were conducted during the Omicron wave.
With both vaccines, there were no cases of myocarditis or pericarditis, conditions that involve inflammation of the heart muscle and surrounding tissue.
While Moderna is testing an Omicron-specific booster, waiting for something like this to be available is not advised because it would be for adults first and would need to go through all the necessary safety and regulatory steps, which means it could be a long time before something like that is available for children. But if younger children are vaccinated now with the current vaccines, any new boosters or vaccines developed down the road could be used to boost the protection they already have, according to experts.
The FDA said that, based on experience with adults, a booster dose may be needed in children under five as well. Moderna has begun testing booster shots in all pediatric age groups.
New Omicron variant reported in India
The ministry’s Corona commissioner Prof. Salman Zarka said in an online briefing for health reporters on Wednesday that a new sub-type of the Omicron variant has been reported in India and other countries and that it is being followed carefully by experts. Called BA.75, it seems to be more infectious than previous sub-types.
Zarka urged that people who return from trips abroad, especially to India and Africa – wear masks whenever possible, including in planes and airports, and that those from countries with BA.75 undergo institutional PCR tests rather than less-specific and -accurate home antigen tests to help the ministry identify where the sub-type is thriving.
He added that with Eid-al-Adha, the Muslim “Holiday of the Sacrifice) –honoring the willingness of Ibrahim (Abraham) to sacrifice his son Ismail (Ishmael) as an act of obedience to Allah’s command – begins on Saturday night and ends of Wednesday, many will go to prayers in mosques and family dinners. He urged that participants undergo antigen tests before going to family gathers if elderly will attend and to wear masks as much as possible.
Zarka noted that 90% of all those hospitalized in serious condition now –which has grown to 471, plus 14 dead in the past seven days – are aged 60 and over or suffer from chronic diseases at younger ages.
While the ministry has not yet decided to require everyone to wear masks indoors, especially in crowded places, because explaining the ned is better, it is considering such a requirement in the coming weeks. “Discomfort from masks is nothing compared to complications of those who become seriously ill after being infected,” he declared.
As for when babies and young children will be able to get vaccines, Zarka said that the ministry is in the process of purchasing the special low doses and they will arrive soon for all parents who want them.
